Our Science
Our Science
Targeting multiple pathways of tumour immune resistance
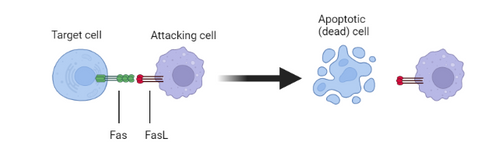
Fas/FasL induces apoptotic cell death
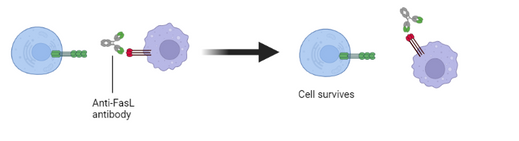
The interaction of FasL with its receptor, Fas, results in apoptotic cell death of the Fas-bearing cell. This fundmental mechanism of cell death is very well controlled in health. It is regulated both by the tightly restricted expression of FasL and by the sensitivity of cells to FasL signalling, through control of the expression of Fas, the signal transduction machinery associated with Fas and inhibitors of the process. Dysregulation of this control in disease leads to inappropriate cell death and severe health consequences. Our therapeutic antibodies bind strongly to FasL and potently prevent cell death by this mechanism.
Eye Diseases
The involvement of FasL in the death of cells of the retina provides a compelling rationale for FasL blockade as a treatment for retinal disease. FBP001 is our candidate therapeutic antibody for eye diseases and has been designed specifically for intravitreal therapy. The intravitreal delivery of therapeutic antibodies is a common and well-accepted treatment and FBP001 can provide potent and lasting blockade of FasL while minimising systemic exposure.
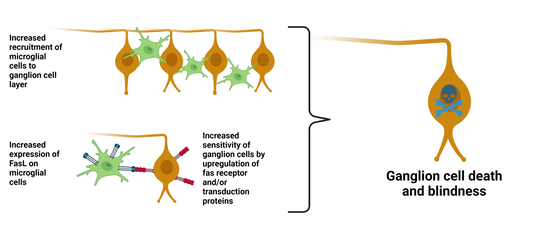
Glaucoma is an optic neuropathy characterized by retinal
ganglion cell (RGC) death and consequent irreversible loss of vision. Although increased ocular pressure associated with the disease can be controlled, many patients still suffer progressive visual field loss. Inhibition of RGC death therefore represents a key goal in the treatment of glaucoma.
FasL is expressed in glaucoma on, for example, microglial cells and has been shown to be a major mediator of RGC cell death.
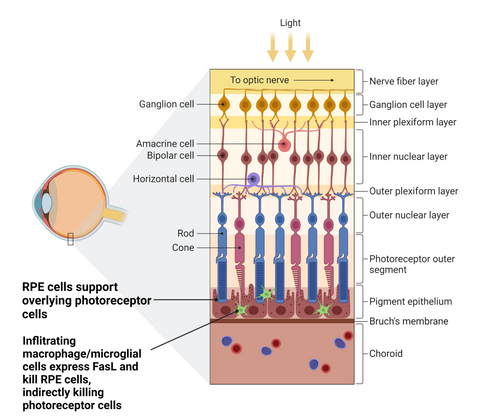
Dry Age-related Macula Degeneration (dry AMD) is a progressive disease affecting the central portion of the retina and is a significant cause of cause of vision loss in ageing populations.
Both photoreceptor cells and retinal pigmented epithelial (RPE) cells (upon which the photoreceptor cells rely for support) can become sensitive to FasL-induced apoptosis. Late stage dry AMD patients display areas of RPE cell death known as geographic atrophy, which leads to death of overlying photoreceptor cells and consequent blindness. There is a high medical need for treatments that can slow or stabilise this disease.
Cancer
T-cells are a major weapon in the immune system’s fight against cancer. As they encounter tumour antigens, T-cell populations reactive to those antigens activate and expand. Unfortunately the more activated (and therefore effective) the T-cell becomes, the more sensitive it becomes to FasL-mediated death. As a result these effective T-cell populations are eliminated before they can destroy tumour cells. Thus, blocking this T-cell deletion with an anti-FasL antibody leads to more antigen-reactive cells with tumour-fighting potential and provides a novel anti-cancer therapy either alone or, depending on the particular setting, in combination with other agents that can stimulate T-cell activity. FBP002 is our clinical development candidate for cancer indications and is a humanised monoclonal antibody that potently binds to, and neutralises, FasL. FBP002 has been engineered to provide extended duration of action after intravenous administration
.
Colorectal Cancer
Our first target cancer indication is a large subset of metastatic colorectal cancer where there is high unmet medical need and compelling evidence that FasL-mediated T-cell depletion is a major mechanism of immune resistance